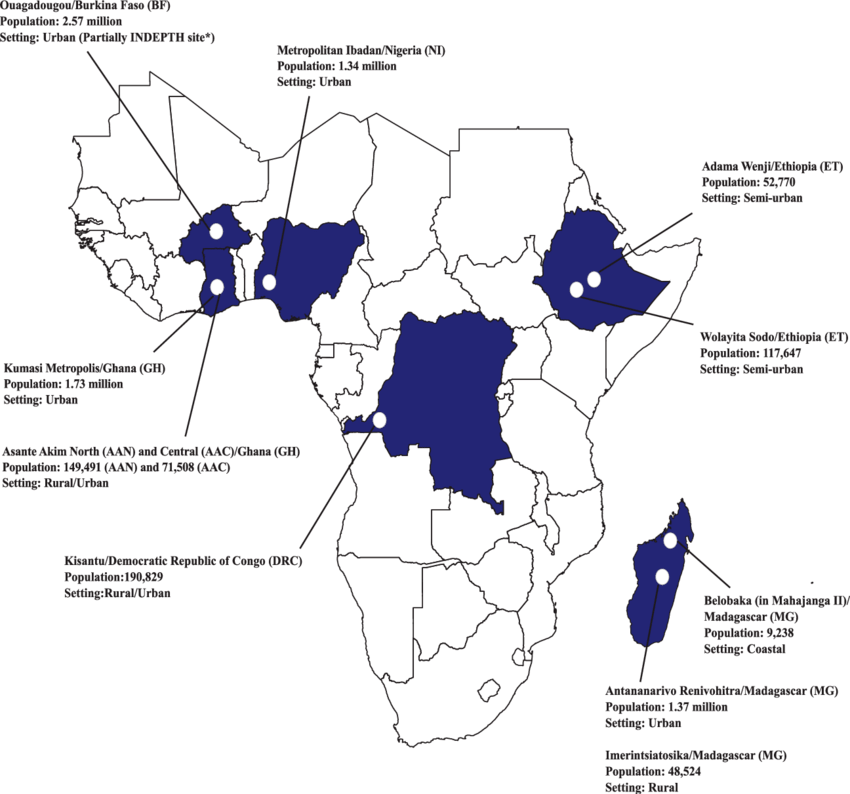
Locations of the Severe Typhoid Fever in Africa program sites. Population data sources: Burkina Faso: United Nations population division, Department of Economic and Social Affairs, 2014; Ouagadougou Health and Demographic Surveillance System routine data, 2015 (Niokoll/Polesgo nested in Ouagadougou); International Network for the Demographic Evaluation of Populations and Their Health. Democratic Republic of Congo: Kisantu Central Health Zone Office, 2017 (Nandu/Kavwaya nested in Kisantu). Ethiopia: 2016 Ethiopia Health Management Information System, Ministry of Health. Ghana: Agogo Presbyterian Hospital official catchment area (Asante Akim North); 2010 Population and Housing Census (Asante Akim Central and Kumasi Metropolis). Madagascar: Ministry of Health, Repartition de la population par Fokotany, 2018 (Imerintsiatosika); Madagascar Population Statistics from Institut National de la Statistique de Madagascar (National Institute of Statistics)/United Nations Population Fund, 2015 (Antananarivo); commune census, 2019 (Belobaka).
Nigeria: Annual Abstract of Statistics 2011, National Bureau of Statistics, Federal Republic of Nigeria. Abbreviation:
INDEPTH, International Network for the Demographic Evaluation of Populations and Their Health.
Source publication
The Severe Typhoid Fever in Africa Program: Study
… on the Typhoid Fever Surveillance in Africa Program
(TSAP) network [6], the SETA program utilized and expanded on previously established fever surveillance infrastructure in sub-Saharan Africa. Six countries were selected exhibiting high disease endemicity (Burkina Faso, Ghana, Madagascar), further need for in-country investigations on TF (Ethiopia), and value of extending to additional study sites to countries with large population numbers (Democratic Republic of Congo and Nigeria); study sites in these countries have been integrated into the SETA program to enable harmonized multicountry surveillance and data comparability (Figure 1). Each SETA site has two distinct study areas (Table 2): a medically served area where surveillance and subsequent studies (case follow-up, enrollment and follow-up of neighborhood controls [NCs] and household contacts [HCs], healthcare utilization survey, and cost of illness and long-term socioeconomic studies) were performed, and a medically underserved area where the frequency of mortality due to suspected severe TF was assessed through postmortem questionnaires…
Context 2
… on the Typhoid Fever Surveillance in Africa Program
(TSAP) network [6], the SETA program utilized and expanded on previously established fever surveillance infrastructure in sub-Saharan Africa. Six countries were selected exhibiting high disease endemicity (Burkina Faso, Ghana, Madagascar), further need for in-country investigations on TF (Ethiopia), and value of extending to additional study sites to countries with large population
While data are limited, case reports and case series suggest that the burden of TIP in sub-Saharan Africa is highly variable and may be substantial in certain settings [5,11, 12]. The Severe Typhoid in Africa Program (SETA), conducted in 6 countries, investigated bacterial etiologies of fever, including severe complications
[13]. Using the SETA data, our goal was to describe the epidemiology, clinical profiles, and associated risk factors for nontraumatic IPs in children and adults, and to categorize these cases based on their likelihood of being TIP…
… This study included participants enrolled in the
SETA program in 6 countries (Burkina Faso, Democratic Republic of Congo [DRC], Ethiopia, Ghana, Madagascar, and Nigeria); detailed methods of the study have been previously published [13]. Patients of all ages residing in the defined catchment areas within each of the participating countries meeting fever criteria (defined as having a temperature of ≥37.5°C axillary or ≥38.0°C tympanic measurement upon examination or a history of 3 consecutive days of self-reported fever within the preceding 7 days) were eligible for inclusion in the surveillance study and were evaluated for bacteremia by blood culture diagnostics…
… Blood agar was incubated at 5% carbon dioxide and other media were incubated in ambient conditions at 37° C for 18-24 hours. Further details on microbiological confirmation of S. Typhi have been published previously.
Characterization of Typhoid Intestinal Perforation Due to the co-occurrence of S. Typhimurium and S. Enteritidis in settings where iNTS disease occurs, standalone S. Typhimurium and S.
Enteritidis vaccines have never gained traction from a global health perspective. Meanwhile, typhoid fever is increasingly appreciated as a problem in Africa from recent pan-African surveillance studies, in particular the Typhoid Surveillance in Africa Program (TSAP) [65] and the Severe Typhoid in Africa (SETA) study [66], These 2 studies examined typhoid fever incidence in sites in Africa where typhoid fever has been reported to be a problem. Separately, the outbreak potential of typhoid fever makes a strong case for adding a licensed TCV to bivalent NTS vaccine combinations.
Salmonella Combination Vaccines: Moving Beyond